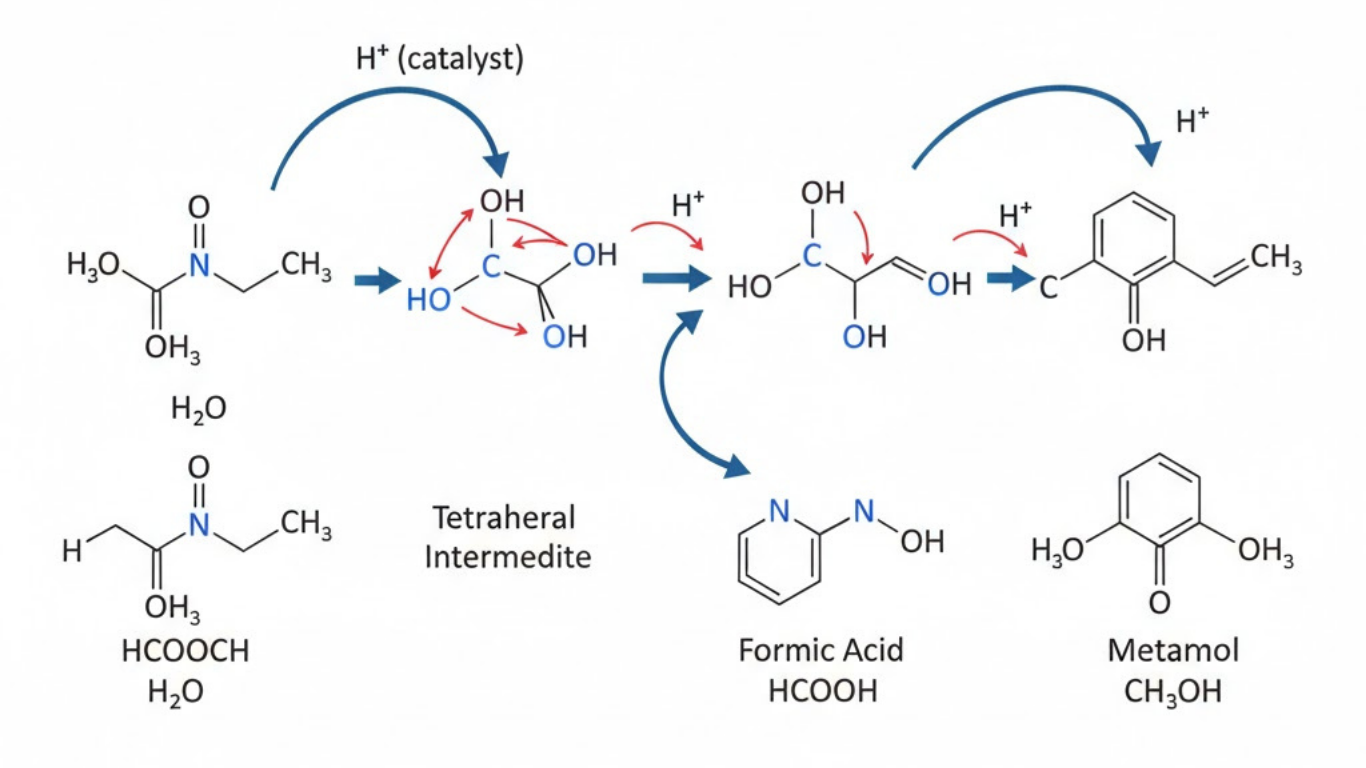
Ester hydrolysis is one of the most essential reactions in organic chemistry, bridging fundamental principles of molecular structure with real-world applications in biology, industry, and environmental science. A commonly studied example involves the breakdown of esters such as HCOOCH in the presence of H₂O to yield corresponding acids and alcohols. Although CH₂ is not a direct reactant in this reaction, it often appears in structural formulas, mechanistic diagrams, and organic frameworks that help explain how the transformation takes place. Understanding how HCOOCH interacts with water provides insight into the nature of ester bonds, reaction mechanisms, catalysis, and the broader chemistry of organic molecules.
This article explores the chemistry behind the reaction involving HCOOCH, the role of H₂O as a reactant, the significance of CH₂ groups in organic structures, and why this hydrolysis reaction remains a foundational concept for students and professionals in chemistry.
1. Understanding the Ester HCOOCH
The molecule HCOOCH is commonly used as shorthand for an ester resembling methyl formate (HCOOCH₃), though written here in a simplified keyword form. Esters are characterized by the functional group:
R–CO–O–R′
In the case of an ester like HCOOCH, the structure can be interpreted as containing:
-
A formyl group (HCO–) on one side
-
An alkoxy fragment (–OCH…) on the other
The nature of this bond makes the molecule especially susceptible to hydrolysis when exposed to water. Esters frequently participate in reactions in which the carbon–oxygen bond is cleaved, especially in acidic or basic environments.
Even though the keyword CH₂ does not specifically appear within HCOOCH, CH₂ groups are foundational building blocks in many ester derivatives. Long-chain esters, lipids, and synthetic polymers feature repeated CH₂ units, and understanding their reactivity helps explain why ester hydrolysis plays such a critical role in the breakdown of biological molecules and industrial materials.
2. The Role of H₂O in Ester Hydrolysis
In the hydrolysis reaction, H₂O acts as a nucleophile—an electron-rich species that attacks the electron-deficient carbonyl carbon of the ester. The general reaction for ester hydrolysis can be written as:
Ester + H₂O → Carboxylic Acid + Alcohol
When HCOOCH reacts with H₂O, the ester bond breaks, producing a formic-acid derivative and an alcohol depending on the R′ group attached. This transformation may occur spontaneously under certain conditions, but more often it is catalyzed by either acids or bases.
Why H₂O is important in hydrolysis
Water is abundant, stable, and capable of participating in both acidic and basic environments. Its amphoteric nature allows it to:
-
Accept protons (acting as a base)
-
Donate protons (acting as an acid)
-
Form hydrogen bonds
-
Serve as a solvent that stabilizes transition states
These properties make H₂O not only a reactant but also a medium that facilitates the entire hydrolysis process.
3. Acid-Catalyzed Hydrolysis of HCOOCH
In acidic conditions, ester hydrolysis proceeds through a protonation-driven mechanism. The carbonyl oxygen of HCOOCH is protonated by an acid, increasing the electrophilicity of the carbonyl carbon. Water (H₂O) then attacks this carbon, forming a tetrahedral intermediate that eventually collapses, releasing an alcohol and forming the carboxylic acid.
Mechanism overview
-
Protonation of the carbonyl oxygen increases reactivity.
-
Nucleophilic attack by H₂O generates an intermediate.
-
Proton transfers help stabilize the structure.
-
Cleavage of the C–O bond releases the alcohol fragment.
-
Deprotonation yields the carboxylic acid.
This route is reversible, meaning esterification (the opposite of hydrolysis) can occur if water is removed or alcohol is present in large excess.
4. Base-Catalyzed Hydrolysis (Saponification)
In contrast, base-catalyzed hydrolysis is irreversible. Hydroxide ions (OH⁻), far stronger nucleophiles than water molecules alone, directly attack the carbonyl carbon of HCOOCH. This attack forms a tetrahedral intermediate that collapses to yield a carboxylate ion and an alcohol.
Why the reaction is irreversible
The carboxylate ion is highly stabilized and cannot regenerate the ester under basic conditions. This principle is used industrially in the production of soaps, detergents, and fatty acid salts.
5. The Significance of CH₂ in the Reaction Context
Although CH₂ is not a reactant in the hydrolysis of HCOOCH, it is an essential structural component in a vast range of esters and organic chains. Understanding the behavior of CH₂ groups helps contextualize hydrolysis in real systems. For example:
-
Fats and oils contain long chains of CH₂ units and ester linkages, all susceptible to hydrolysis.
-
Polymers featuring repeating CH₂ groups undergo environmental degradation through ester cleavage.
-
Biomolecules such as phospholipids use ester bonds adjacent to CH₂-rich hydrophobic tails.
By studying the simple reaction between HCOOCH and H₂O, chemists gain a foundation for understanding more complex CH₂-containing molecules.
6. Thermodynamics and Kinetics of the Reaction
The hydrolysis of HCOOCH with H₂O follows general thermodynamic and kinetic principles seen across ester chemistry.
Thermodynamically
-
The products (carboxylic acid and alcohol) are often more stable.
-
Hydrogen bonding with H₂O stabilizes reaction intermediates.
-
Base hydrolysis is strongly favored due to the formation of stable carboxylate ions.
Kinetically
-
Acidic catalysis increases electrophilicity.
-
Basic catalysis increases nucleophilicity.
-
Temperature, solvent polarity, and steric hindrance all influence the rate.
Because HCOOCH is small and relatively unhindered, its reaction with H₂O occurs more quickly than hydrolysis of bulkier esters.
7. Applications of Ester Hydrolysis
The reaction involving HCOOCH and H₂O is a model for countless real-world processes.
Biological relevance
-
Digestion of dietary fats via lipase-catalyzed hydrolysis
-
Breakdown of lipid membranes during metabolism
-
Cleavage of ester-linked neurotransmitters, such as acetylcholine
Industrial significance
-
Soap production (saponification of fatty esters)
-
Manufacturing of plastics that require controlled hydrolysis
-
Synthesis of fragrances and flavors that are esters or rely on ester formation and breakdown
Environmental chemistry
-
Hydrolysis determines the biodegradability of plastics and organic pollutants.
-
Many pesticides undergo hydrolytic decomposition in soil and water.
-
Environmental aging of materials often results from ester bond cleavage.
Even though the example reaction uses the simplified keyword forms HCOOCH, CH₂, and H₂O, the principles extend far beyond this simple equation.
8. Mechanistic Importance in Organic Chemistry
Studying the hydrolysis of HCOOCH with H₂O helps build fundamental skills:
-
Recognizing nucleophile–electrophile interactions
-
Understanding reaction intermediates
-
Distinguishing between acidic and basic pathways
-
Predicting product formation
-
Applying knowledge to larger, more complex molecules
The role of CH₂ groups helps students envision how hydrolysis applies to long carbon chains and natural biomolecules.
9. Stability and Structure: Why HCOOCH Reacts Easily
Several features make HCOOCH particularly reactive toward H₂O:
-
The formyl group (HCO–) strongly withdraws electron density, increasing carbonyl reactivity.
-
The small molecular size reduces steric hindrance.
-
The carbonyl carbon is highly accessible for nucleophilic attack.
In comparison, esters with bulky CH₂-rich substituents may hydrolyze more slowly due to steric shielding. However, CH₂ groups also influence hydrophobic interactions and solubility, factors that indirectly affect hydrolysis rates in biological and environmental systems.
10. Final Products of Hydrolysis
The hydrolysis process typically produces:
1. A carboxylic acid
Derived from the HCO– portion of the ester, this acid retains strong hydrogen bonding capabilities and participates in many secondary reactions.
2. An alcohol
Released from the alkoxy portion of HCOOCH, the alcohol may vary depending on the specific ester structure.
These products often serve as intermediates in:
-
Organic synthesis
-
Metabolic pathways
-
Polymer degradation
-
Flavor and fragrance formation
Conclusion
The reaction involving HCOOCH, CH₂, and H₂O serves as a powerful example of ester hydrolysis, demonstrating fundamental chemical principles that underpin vast areas of organic chemistry. Through the nucleophilic attack of water on an ester, the molecule breaks apart into a carboxylic acid and an alcohol—an elegant yet essential transformation.